Abstract
Myelodysplastic syndromes (MDS) are clonal, myeloid malignancies that emerge and progress due to the expansion of disease-initiating aberrant hematopoietic stem cells that can evolve into Acute Myeloid Leukemia (AML). FDA approved therapies such as the recently approved Bcl-2 inhibitor venetoclax, FLT3 inhibitors, among others, have moved the field forward in newly diagnosed MDS/AML. However, relapsed/refractory (R/R) disease, as well as leukemic transformation post-MDS continues to have a poor prognosis. A pool of hematopoietic stem and progenitor cells (HSPCs) escape chemotherapy, proliferate during disease remission, and causes relapse partly in effect due to signaling effector mutations. It is imperative, for future therapeutic agents, to target these HSPCs populations to achieve a durable remission for aggressive myeloid malignancies. There is an urgent need to develop mouse models that recapitulate human disease for the study of pathogenesis and drug development in these disorders.
Signal transducer and activator of transcription 3 (STAT3) belongs to the STAT family of transcription factors that are inappropriately activated in several malignancies. Our preliminary data indicates that STAT3 is overexpressed in MDS and AML stem cells and is associated with an adverse prognosis in a large cohort of patients. (Shastri et al, JCI 2018). We have successfully demonstrated that a selective antisense oligonucleotide inhibitor of STAT3, Danvatirsen, is rapidly incorporated into MDS/AML HSPCs and induces selective apoptosis and downregulation of STAT3 in these cells in comparison with healthy control HSPCs.
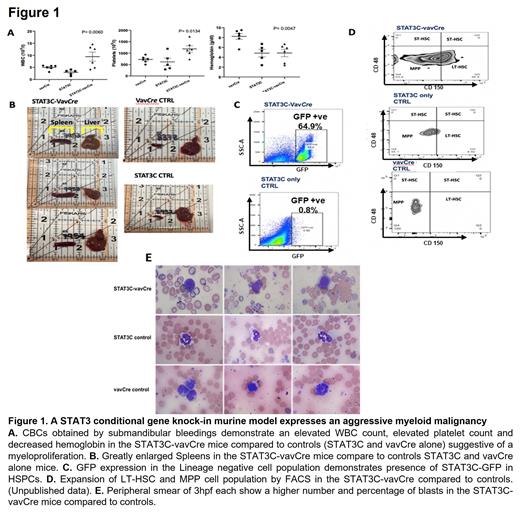
To determine the role of STAT3 in the initiation of myeloid malignancies, a murine model was generated by crossing R26STAT3C stopfl/fl mice with vavCre transgenic mice. In this model, a hyperactive version of STAT3, STAT3C, is knocked into the Rosa26 locus with an upstream floxed stop cassette (R26STAT3C stopfl). Excision of the stop cassette by Cre recombinase leads to expression of a flag-tagged STAT3C protein and concomitant expression of EGFP in hematopoietic cells. GFP expression allows tracking of cells in which the floxed stop/Neo cassette is deleted and STAT3C is expressed. STAT3C-vavCre double transgenic mice were validated by GFP expression in HSPCs and differentiated hematopoietic cells. The STAT3C-vavCre mice developed ruffled fur, a hunched phenotype and weight-loss by five months of age. CBC analysis of STAT3C-vavCre mice shows a proliferative phenotype reminiscent of high-risk MDS/AML with higher WBC & platelet counts and lower hemoglobin (Figure 1A). Review of the peripheral smear showed an increase in granulocytic precursors that are likely leukemic blasts (Fig 1E). In addition, STAT3C-vavCre mice developed massive splenomegaly (Figure 1B). HSC lineage analysis by FACS showed the presence of GFP positive cells (Figure 1C) with increased expansion of the MPP and HSC compartment compared to controls, suggesting a stem and progenitor phenotype (Figure 1D). Murine myeloid colony assays showed larger colonies in the STAT3C-vavCre mice compared to controls. At this time, single cell RNA sequencing, and bulk RNA sequencing are being performed and will be used to further characterize the phenotype of the STAT3C-vavCre transgenic mice in addition to bone marrow and splenic aspirates & biopsies.
Through the generation of a STAT3C-vavCre mouse model, that recapitulates the features of MDS/AML, we aim to further our understanding of the molecular mechanisms and pathways that play an important role in MDS to AML transformation and will help us identify downstream mediators of this event that can be therapeutically targeted. We would also like to use this murine model as an ideal substrate for preclinical studies of STAT3 targeting therapies in hematologic malignancies such as previously reported antisense inhibitors of STAT3 and STAT3 degraders.
Frank: Roche Genentech: Research Funding; Kymera: Consultancy, Research Funding; Revitope: Consultancy; Vigeo: Consultancy. Verma: Throws Exception: Current equity holder in publicly-traded company; BMS: Research Funding; GSK: Research Funding; Acceleron: Consultancy; Incyte: Research Funding; Stelexis: Current equity holder in publicly-traded company; Medpacto: Research Funding; Curis: Research Funding; Eli Lilly: Research Funding; Celgene: Consultancy; Stelexis: Consultancy, Current equity holder in publicly-traded company; Novartis: Consultancy. Shastri: Kymera Therapeutics: Research Funding; GLC: Consultancy; Guidepoint: Consultancy; Onclive: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal